Abstract
Background: Treatment outcomes in patients (pts) with AML and HR-MDS who progress after HMA +/- venetoclax (VEN) based regimen remains poor and warrants new therapeutic strategies. CPX-351 (Vyxeos™) is a dual liposomal formulation of cytarabine and daunorubicin, at a fixed synergistic 5:1 molar ratio, approved by US Food and Drug Administration (FDA) for the treatment of newly diagnosed therapy-related AML or AML with myelodysplastic related changes (AML MRC). GO (Mylotarg™) is a humanized IgG4 antibody directed against CD33 and conjugated to the DNA toxin calicheamicin and approved by the FDA for the treatment of newly diagnosed or R/R CD33-expressing AML. We hypothesized that the combination of CPX-351 and GO could be synergistic in this high risk pt population and have potent anti-leukemic activity.
Aim: To determine the safety and efficacy of CPX-351 in combination with GO in patients with R/R AML or post-HMA failure HR-MDS.
Methods: We present here the updated results of the Phase 2 single-arm pilot study (NCT03672539) of CPX-351 and GO (CPX-GO) combination therapy in pts with CD33 positive R/R AML or post-HMA failure HR-MDS.
Pts received induction cycle (C) CPX-351 (daunorubicin 44 mg/m2 and cytarabine 100 mg/m2) administered via intravenous (IV) infusion on days (D) 1, 3, and 5. GO was administered at a dose of 3 mg/m2 (capped at 4.5 mg) IV on D1. Pts not attaining complete remission (CR) or CR with incomplete count recovery (CRi) after C1, could receive a 2nd induction cycle of CPX at the same dose, but only on D1 and D3 with GO 3 mg/m2 on D1. Pts attaining CR/CRi could receive up to 2 consolidation cycles, after a minimum of 4 weeks from the start of the previous cycle with CPX (daunorubicin 29 mg/m2 and cytarabine 65 mg/m2) IV on D1 and D3 and GO at 3 mg/m2 on D1. GO was only administered during the second consolidation cycle if there was evidence of measurable residual disease (MRD+) as measured by flow cytometry. GO could also be administered as a single agent for maintenance treatment on D1 every 6 weeks, in case of persistent detection of MRD. CD33 genotyping to assess for GO sensitivity was available for a subset of pts. Response was denoted as per the ELN 2017 criteria for AML and IWG 2018 criteria for MDS.
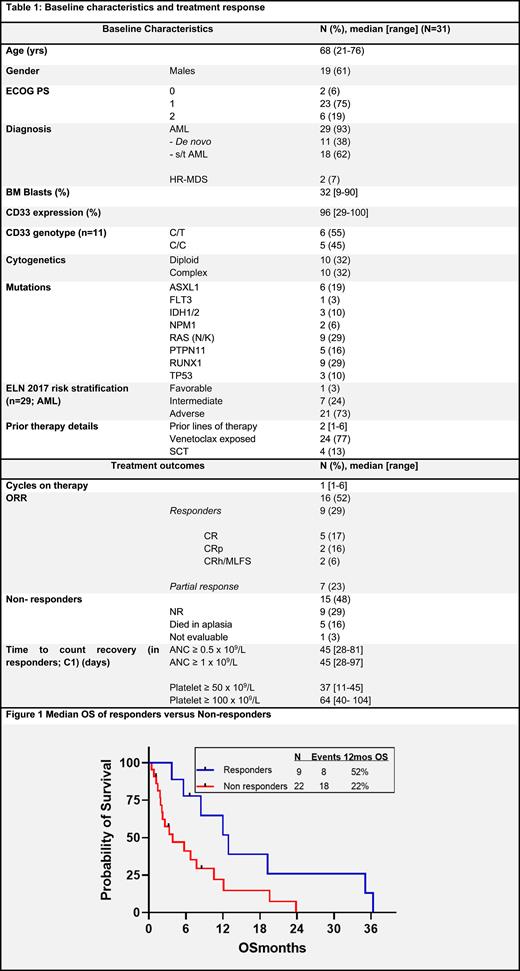
Results: From Nov 2018- Apr 2022, 31 pts were treated on the trial, 29 (93%) pts with AML and 2 pts (7%) with HR-MDS. Baseline disease, treatment and response characteristics are mentioned in Table 1. The median lines of prior AML therapy were 2 (range, 1-6). Eleven pts (38%) had baseline de novo AML, while 15 (52%) and 3 (10%) pts had secondary (s-AML) and therapy-related (t-AML) AML respectively. Twenty-three AML pts (79%) had prior VEN exposure. Both the prior HR-MDS patients had bone marrow (BM) blasts >15% and one pt had prior VEN exposure. Overall, 21 pts with AML (72%) were adverse risk and only 1 pt (3%) had favorable risk AML (diploid karyotype with NPM1 mutation). Four pts (all AML; 13%) had undergone a prior stem cell transplantation (SCT).
The median number of cycles on trial were 1 (range, 1-6). At the time of data cutoff, none of the pts were on active study therapy and the median follow up was not reached. Sixteen pts (52%) had an overall response; 9 pts had CR/CRh/MLFS (8 AML and 1 MDS= responders), after a median of 1 cycle of therapy, and 7 pts had a partial response (all AML). CD33 genotype did not affect response rates (no response in 5 pts with C/C vs 2/6 responders in C/T genotype, p=0.45). Amongst prior VEN exposed pts, 5/24 (21%) responded versus 3/7 (43%) non VEN exposed pts (p=0.15)
One pt could directly proceed to a SCT in CR after the trial therapy. For relapse free survival (RFS) analysis partial responders were grouped under non-responders. The median RFS and overall survival (OS) for the whole cohort was 6.3 and 6.8 months (mos) respectively, and median OS was 12.9 mos for responders(Fig. 1).
One pt had a grade 2 infusion reaction to GO. Overall, adverse events at least possibly attributed to the trial medications were seen in 5 pts, of which 2 events were ≥ grade 3 (mucositis and seizures). No pt had hepatic veno-occlusive. Eight pts (all non-responders) (26%) died on the trial from infections/leukemia progression and there were no deaths in remission.
Conclusion: In a cohort composed predominantly of heavily pre-treated and VEN exposed adverse risk AML and HR-MDS pts, CPX-GO led to overall response (CR/CRh/MLFS/PR) rates of 52% and year-long median survival in responders (CR/CRh/MLFS) with no major non-hematological adverse event.
Disclosures
Kadia:Delta-Fly: Research Funding; PinotBio: Consultancy; cellenkos: Research Funding; Glycomimetics: Research Funding; Regeneron: Research Funding; Agios: Consultancy; Servier: Consultancy; Abbvie: Consultancy, Research Funding; Astex: Honoraria; Iterion: Research Funding; Genentech: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Ascentage: Research Funding; Genfleet: Research Funding; Astellas: Research Funding; AstraZeneca: Research Funding; Amgen: Research Funding; cyclacel: Research Funding; Pfizer: Research Funding; Novartis: Consultancy; JAZZ: Consultancy, Research Funding. Faderl:Jazz Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Sasaki:Otsuka Pharmaceuticals: Honoraria; Pfizer: Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Short:Takeda Oncology: Consultancy, Research Funding; Astellas: Research Funding; Pfizer: Consultancy; Novartis: Consultancy; Amgen: Consultancy, Honoraria; AstraZeneca: Consultancy; Stemline Therapeutics: Research Funding. Daver:Trillium: Consultancy, Research Funding; Trovagene: Research Funding; Gilead Sciences, Inc.: Consultancy, Research Funding; Hanmi: Consultancy, Research Funding; Syndax: Consultancy; Celgene: Consultancy; Jazz: Consultancy; Novimmune: Research Funding; FATE Therapeutics: Research Funding; Glycometrics: Research Funding; Arog: Consultancy; Amgen: Consultancy, Research Funding; Servier: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Daiichi-Sankyo: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Novartis: Consultancy; Pfizer: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Shattuck: Consultancy; Agios: Consultancy. DiNardo:GenMab: Membership on an entity's Board of Directors or advisory committees; Cleave: Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria; Gilead: Honoraria; Forma: Research Funding; Takeda: Honoraria; Astex: Research Funding; Novartis: Honoraria; LOXO: Research Funding; ImmuneOnc: Honoraria, Research Funding; Foghorn: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Bluebird Bio: Honoraria; Astellas: Honoraria; Servier: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Research Funding; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Kura: Honoraria, Membership on an entity's Board of Directors or advisory committees. Ferrajoli:Janssen: Membership on an entity's Board of Directors or advisory committees; Beigene: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding. Jabbour:Bristol Myers Squibb: Other: Advisory Role, Research Funding; AbbVie: Other: Advisory Role, Research Funding; Adaptive Biotechnologies: Other: Advisory Role, Research Funding; Amgen: Other: Advisory Role, Research Funding; Pfizer: Other: Advisory Role, Research Funding; Spectrum: Research Funding; Takeda: Other: Advisory Role, Research Funding; Genentech: Other: Advisory Role, Research Funding. Borthakur:Catamaran Bio, Abbvie, PPD Development, Protagonist Therapeutics, Janssen: Consultancy; Pacylex, Novartis, Cytomx, Bio Ascend: Membership on an entity's Board of Directors or advisory committees; Astex Pharmaceuticals, Ryvu, PTC Therapeutics: Research Funding. Garcia-Manero:Acceleron Pharma: Consultancy; Gilead Sciences: Research Funding; Genentech: Honoraria, Research Funding; Curis: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Aprea: Honoraria; Astex: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding. Andreeff:Syndax: Consultancy, Research Funding; Senti Bio: Consultancy, Research Funding; Chimerix: Current holder of stock options in a privately-held company; Medicxi: Consultancy; Cancer UK: Membership on an entity's Board of Directors or advisory committees; Glycomimetics: Consultancy; Daiichi-Sankyo Inc.: Consultancy, Research Funding; NCI: Membership on an entity's Board of Directors or advisory committees; CLL Foundation: Membership on an entity's Board of Directors or advisory committees; Pinot Bio: Research Funding; Brooklyn ITX: Research Funding; Oxford Biomedical UK: Research Funding; Kintor Pharmaceutical: Research Funding; Reata: Current holder of stock options in a privately-held company; Oncolyze: Current holder of stock options in a privately-held company; Aptose: Consultancy, Membership on an entity's Board of Directors or advisory committees; Leukemia & Lymphoma Society: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Research Funding; Breast Cancer Research Foundation: Research Funding; German Research Council: Membership on an entity's Board of Directors or advisory committees. Kantarjian:Novartis: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Astellas Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Research Funding; Ipsen Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; KAHR Medical Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; NOVA Research: Honoraria; Amgen: Honoraria, Research Funding; Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; ImmunoGen: Research Funding; Daiichi-Sankyo: Consultancy, Research Funding; Pfizer: Honoraria, Research Funding; Takeda: Honoraria. Ravandi:Biomea Fusion, Inc.: Research Funding; Astellas: Consultancy, Honoraria, Research Funding; Astex/Taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Research Funding; BMS/Celgene: Consultancy, Honoraria, Research Funding; Amgen: Honoraria, Research Funding; AstraZeneca: Consultancy; Xencor: Research Funding; Prelude: Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Novartis: Consultancy; Syos: Consultancy, Honoraria, Research Funding. Alvarado:Daiichi-Sankyo/Lilly: Research Funding; Sun Pharma: Research Funding; FibroGen: Research Funding; Jazz Pharmaceuticals: Research Funding; BerGenBio: Research Funding; Astex Pharmaceuticals: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal